EasyMed is among China’s earliest professional teams specializing in guidewire production. Our hydrophilic guidewires boast the top-tier performance across China, backed by our self-owned full production chain and proprietary coating technology.

Founded in March 2025, the Company is a wholly-owned subsidiary of EasyMed Scientific (SHENZHEN) Medical Co., LTD., with a registered capital of HK$10 million.It is located at Room 702, Building 2, Jiawei Photovoltaic Lighting Industrial Park, Pingdi Sub-district, Longgang District, Shenzhen. Dedicated to the R&D and production of high-quality interventional medical consumables, we adhere to the philosophy of "Precision, Wisdom, Inclusiveness and Long-term Development", striving to bring safe and efficient interventional therapy to patients worldwide.
We have the independent R&D capabilities for coating processes and equipment development, and can flexibly meet diverse product requirements, including hydrophilic coating, continuous grinding processes, etc.In terms of quality management, we have established a comprehensive system in strict compliance with international standards such as ISO 13485 and ISO 14971, and plan to obtain the BSI certification in May-June 2026.Meanwhile, we are committed to providing personalized customization and rapid response services, supporting one-to-one demand docking with customers, so as to fully ensure the efficient progress of projects and the high quality of products.

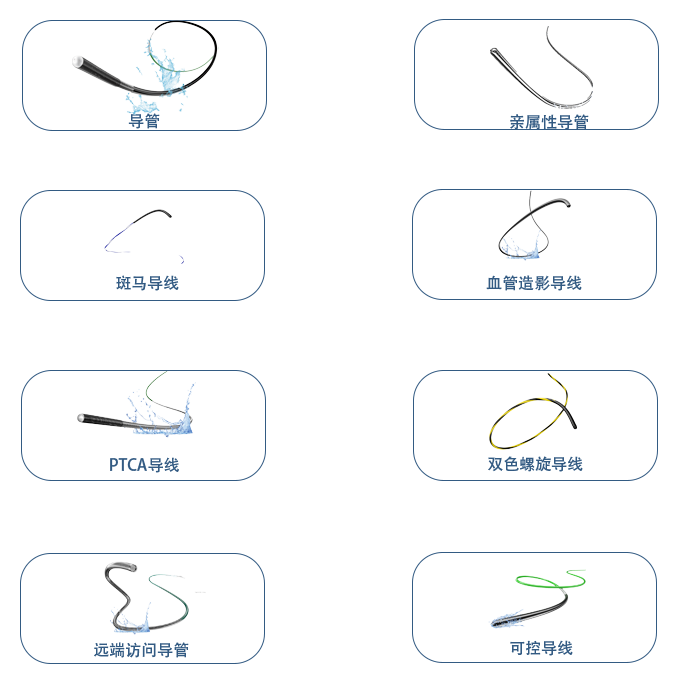
A national high-tech enterprise specializing in the R&D, production and sales of high-end interventional medical catheters. With technological innovation at its core, the company is committed to providing precise and safe catheter solutions for minimally invasive interventional procedures in fields such as cardio-cerebrovascular diseases, oncology and peripheral vascular disorders.
Our company owns a Class 10,000 cleanroom covering an area of approximately 4,000 square meters, integrating the entire production processes of cleaning, extrusion, injection molding, coating and assembly. The annual production capacity of guidewires reaches 4 to 5 million units.Core processes include high-precision hydrophilic coating, precision metal grinding, micro-spring forming and multi-specification guidewire extrusion. The testing system covers physical property evaluation, chemical safety assessment and simulated use verification, providing comprehensive guarantees for product performance and application safety.
